LOXO-292: Bioactivity and Chemicaland toxicological studies
General description
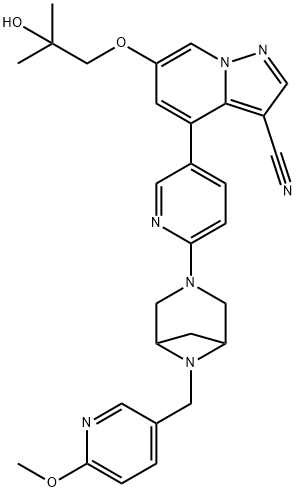
The chemical name of LOXO-292 (Figure 1) is 6- (2-hydroxy-2-methylpropoxy)-4- (6-(6-((6-methoxypyridin-3yl) methyl) -3,6-diazabicyclo[3.1.1] heptan-3-yl) pyridin-3-yl) pyrazolo [1,5-a] pyridine-3-carbonitrile and corresponding to the molecular formula C29H31N7O3. It has a relative molecular mass of 525.61 g/mol. It is a novel broad-spectrum anticancer drug with the targeted function for RET protooncogene and the following structure.[1]
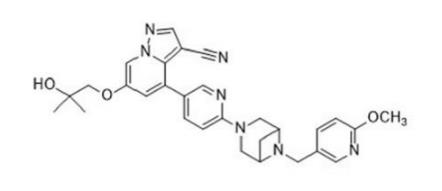
Figure 1 the molecular formula of LOXO-292
Indication
LOXO-292 is a selective and potent RET inhibitor.[2] Currently, it has FDA-approved indications for the treatment of metastatic RET fusion–positive non–small cell lung cancer (NSCLC) and thyroid cancers, as well as it as an alternative therapy to Lenvatinib for metastatic medullary thyroid cancer.[3] Recent evidence has also demonstrated antitumor activity in additional RET fusion–driven advanced solid tumors. However, the implications of genomic alterations outside of RET fusion–positive and RET mutation–positive disease have not been fully characterized. Thus, the safety and efficacy of LOXO-292 has not been fully described in solid tumors with additional RET alterations, specifically those harboring RET amplification.[4]
Pharmacodynamics
In vitro, LOXO-292 inhibited wild-type RET and multiple mutated RET isoforms as well as VEGFR1 and VEGFR3 with IC50 values ranging from 0.92 nM to 67.8 nM. (LOXO-292-PHARM-003, LOXO-292PHARM-021; LOXO-292-PHARM-027). [1] In vivo: In mice implanted subcutaneously with NIH-3T3 cells engineered to express a constitutively active KIF5B-RET fusion protein, single oral doses of LOXO-292 suppressed RET phosphorylation in tumours at single doses of 10 mg/kg and 30 mg/kg showed near complete inhibition of RET kinase activity (LOXO292-PHARM-008). LOXO-292 administered 14 days caused dose-dependent inhibition of tumour growth of KIF5B-RET 3T3 tumours with 62%, 88%, 93% tumour regression when dosed at 10 mg/kg BID, 15 mg/kg BID and 30 mg/kg BID, respectively (LOXO-292-PHARM-006). Twice-daily oral dosing of LOXO-292 caused inhibition of tumour growth in multiple RET-dependent tumour models including those harbouring a V804M gatekeeper mutation (KIF5B-RET-V804M NIH-3T3 and CR2545 CCDC6-RETV804M ) with 16%, 50% and 70% tumour regression when dosed at 10 mg/kg BID, 30 kg/kg BID and 100 mg/kg BID, respectively. The 100 mg/kg BID dosing schedule was not well tolerated as demonstrated by a mean body weight loss of 13.2% (LOXO-292-PHARM-007). Additionally, LOXO-292 also inhibited the growth of two different RET-dependent human cancer cell line mouse xenograft tumour models (TT human MTC cells harbouring an endogenous RET C634W substitution with 20% and 37% at 10 mg/kg BID and 30 mg/kg BID respectively and with 3% and 16 % in LC2/ad human non-small cell lung cancer cells expressing a CCDC6-RET fusion (LOXO-292-PHARM004(GLP)). In Females BALB/c Nude mice implanted with CR2518 patient derived colorectal carcinoma xenograft (PDX) with a CCDC6-RET fusion protein with or without a V804M gatekeeper mutation, selpercatinib showed dose dependent inhibition of tumour growth with 108% and 113% tumour growth inhibition at doses of 10 mg/kg BID and 30 mg/kg BID, respectively (LOXO-292-PHARM-001(GLP)). In Female mice Athymic Nude-Foxn1 implanted with tumour model from CTG-0838 derived from Patient PDX with NSCLC that expresses a KIF5B-RET fusion protein orally (3 or 30mg/kg), LOXO-292 caused dose-dependent inhibition of tumour growth with 82% and 106% tumour growth inhibition at day 25 for the 3 mg/kg BID and 30 mg/kg BID groups, respectively (LOXO-292-PHARM-017(GLP)). This product at orally 30 mg/kg BID inhibited the tumour growth of a RET fusion-positive PDX model implanted directly into the brain and demonstrated a significantly prolonged survival and was well tolerated throughout the 84 days of dosing (LOXO-292-PHARM-013). [1]
Preparation
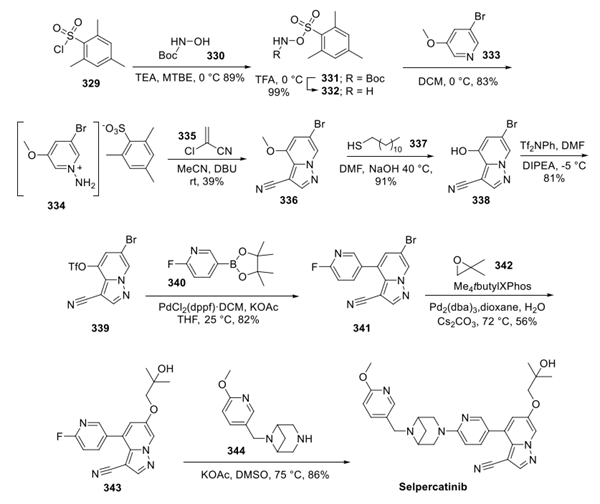
According to the report, there are three synthetic routes to LOXO-292, and all routes include three steps with high, but likely unoptimized, Pd loadings. The following route is most convergent, but the selectivity for coupling of 57 with 56 was not disclosed. The synthesis started with reaction of 3,5-dibromopyridine (1) with (Omesitylsulfonyl) hydroxylamine to provide 2 in 82% yield. Conversion to pyrrolo-pyridine 3 involved reaction with acrylonitrile with DDQ and i-Pr2NEt in dioxane, affording 3 in 52% yield. Cross-coupling of 3 with 4 provided 5 in 68% yield after isolation by chromatography. Bromide to hydroxyl conversion was carried out in a single pot by first formation of the boronate ester followed by addition of base and hydrogen peroxide to oxidize the boronate ester to 7 in 76% yield. Reaction with 2,2-dimethyloxirane afforded 9 in 84% yield. Conversion to LOXO-292 was then performed in two steps, as outlined in Figure 2. [5]
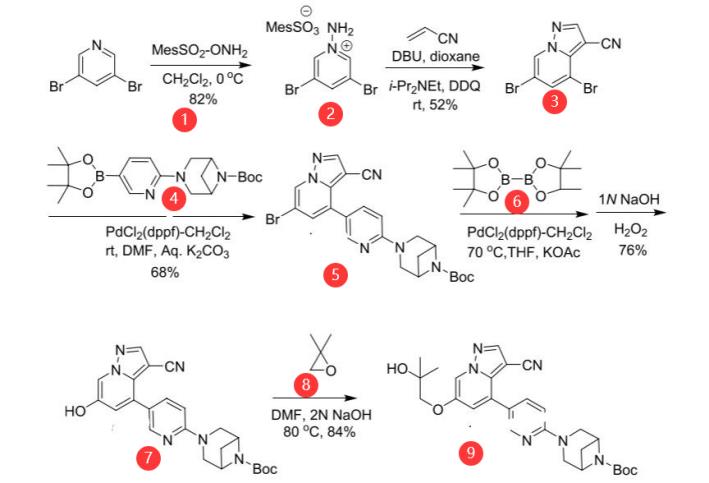
Figure 2 Preparation of LOXO-292
Another synthetic route to LOXO-292 is that compound 1 was functionalized first by conversion to boronate ester 2 in 62% yield after crystallization from EtOAc/n-heptane. Oxidation was performed with N-methylmorpholine N-oxide in THF at 50 °C. Upon completion of the reaction, water was added to crystallize 3, which was isolated in 86% yield. Reaction with 2,2-dimethyloxirane in THF/2NNaOH at 60 °C provided 4, which was isolated in 75% from the reaction mixture after addition of water. Removal of the methoxy group was accomplished with n-dodecanethiol and 50% NaOH in dimethylacetamide at 60 °C. Direct isolation without workup was carried out by addition of the reaction mixture to aqueous citric acid to crystallize 5, which was isolated in 81% yield. Conversion to triflate 6 followed by cross-coupling with 7 afforded 8 in 45% for the two steps, which was then converted to LOXO-292, as outlined in Figure 3. [5]
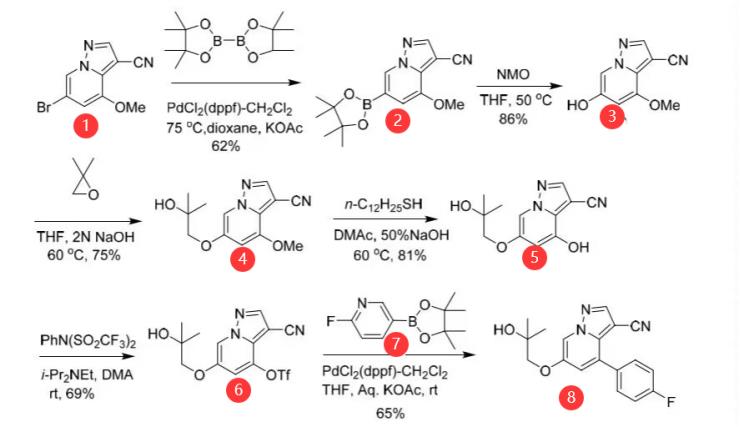
Figure 3 Another Preparation of LOXO-292
Toxicity
LOXO-292 displayed toxicity in both rats and minipigs with large target organs and tissues. Finding were in the haemtopoietic system, lymphoid system, gastrointestinal system (tongue in rat only), pancreas, skeletal system (epiphyseal growth plate of femur/sternum) and reproductive system. [1]
References
1. Assessment report EMA/9037/2021, European Medicines Agency, 2021.
2.Wang W, Shi L, Jin L, et al. Determination of selpercatinib, a RET kinase inhibitor, in rat plasma and its application to a pharmacokinetic study [J]. Biomedical Chromatography.
3.Contreras P C, Montalban M C, Moreno T A, et al. Selpercatinib as an alternative therapy to Lenvatinib for metastatic medullary thyroid cancer. A case report [J]. Endocrine Abstracts, 2021.
4.Chen A , Cameron C, Katherine M, et al. INNV-11. COMPLETE RESPONSE TO SELPERCATINIB IN A PATIENT WITH RECURRENT GLIOBLASTOMA AND RET AMPLIFICATION[J]. Neuro-Oncology, 2021;20( ):966-971.
5. Hughes D L. Review of Synthetic Routes and Crystalline Forms of the Oncology Drugs Capmatinib, Selpercatinib, and Pralsetinib [J]. Organic process research & development, 2021(10):25.
);You may like
Related articles And Qustion
See also
Lastest Price from LOXO-292 manufacturers

US $0.00-0.00/kg2024-05-31
- CAS:
- 2152628-33-4
- Min. Order:
- 1kg
- Purity:
- >99.5% by HPLC
- Supply Ability:
- 100kg/month
US $2.00/kg2024-05-31
- CAS:
- 2152628-33-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 30KG/M